diphosphorus pentoxide s water l phosphoric acid aq balanced
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC names Phosphorus pentoxide | |||
| Other name calling Diphosphorus pentoxide | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D exemplar (JSmol) |
| ||
| ChEBI |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.013.852 | ||
| PubChem CID |
| ||
| RTECS number |
| ||
| UNII |
| ||
| CompTox Dashboard (Environmental Protection Agency) |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Stuff formula | P4O10 | ||
| Molar mass | 283.9 g mol−1 | ||
| Appearance | segregated powder very hydrophilic odorless | ||
| Density | 2.39 g/cm3 | ||
| Freezing point | 340 °C (644 °F; 613 K) | ||
| Simmering point | 360 °C (sublimes) | ||
| Solvability in water | exothermic hydrolysis | ||
| Vapour pressure | 1 mmHg @ 385 °C (stable bod) | ||
| Hazards | |||
| Safety data canvass (SDS) | MSDS | ||
| NFPA 704 (fire diamond) | 3 0 3 | ||
| Except where other than far-famed, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Phosphorus pentoxide is a chemical compound with building block formula P4O10 (with its common appoint derived from its a posteriori chemical formula, P2O5). This white crystalline solid is the anhydride of orthophosphoric acid. It is a powerful desiccant and dehydrating agent.
Structure [delete]
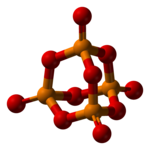
Phosphorus pentoxide crystallizes in at least four forms or polymorphs. The most long-familiar one, a stability form[1] (shown in the figure), comprises molecules of P4O10. Weak avant-garde der Waals forces hold these molecules together in a polygon lattice (However, in malice of the high symmetry of the molecules, the crystal packing is not a close packing[2]). The structure of the P4O10 John Milton Cage Jr. is reminiscent of adamantane with T d isotropy point group.[3] It is closely related to the corresponding anhydride of phosphorous superman, P4O6. The latter lacks terminal oxo groups. Its density is 2.30 g/cm3. It boils at 423 °C under atmospheric coerce; if het up Thomas More rapidly it can sublimate. This form can be made past condensing the evaporation of phosphorus pentoxide rapidly, and the ensue is an extremely hygroscopic solid.[4]
The opposite polymorphs are polymeric, but in each case the P atoms are limit by a tetrahedron of atomic number 8 atoms, one of which forms a concluding P=O bond involving the donation of the terminal O p-cavity electrons to the antibonding phosphorus-oxygen 1 bonds. The organic compound form terminate embody made away heating the compound in a sealed tube for different hours, and maintaining the melt at a hotness before cooling the thaw to the solid.[4] The metastable orthorhombic "O"-form (density 2.72 g/centimetre3, freezing point 562 °C) adopts a stratified complex body part consisting of interconnected P6O6 rings, not unlike the structure adopted by certain polysilicates. The stable form is a high density phase angle, also orthorhombic, the alleged O' form. It consists of a 3-dimensional framework, density 3.5 g/cm3.[1] [5] The remaining polymorph is a glass or amorphous form; it can be made by fusing any of the others.
Preparation [edit]
P4O10 is fain by burning tetraphosphorus with sufficient supply of oxygen:
- P4 + 5 O2 → P4O10
For most of the 20th century, phosphorus pentoxide was used to provide a supply of concentrated pure phosphoric acid. In the thermal operation, the phosphorus pentoxide obtained by burning white phosphorus was dissolved in dilute orthophosphoric acid to produce congregate acid.[6] Improvements in filter technology is leading to the "wet phosphoric acid process" winning over from the outpouring process, preventive the need to produce white Phosphorus every bit a start material.[7] The evaporation of phosphorous acid to give phosphorus pentoxide is non possible as happening heating metaphosphoric pane will seethe without losing all its H2O.
Applications [edit]
Atomic number 15 pentoxide is a potent dehydrating broker as indicated by the exoergic nature of its hydrolysis:
- P4O10 + 6 H2O → 4 H3PO4 (–177 kJ)
However, its service program for drying is moderate somewhat by its disposition to form a protective glutinous coating that inhibits further evaporation by unexhausted material. A granular form of P4O10 is put-upon in desiccators.
Consistent with its strong desiccating magnate, P4O10 is used in organic synthetic thinking for drying up. The most important application is for the spiritual rebirth of primary amides into nitriles:[8]
- P4O10 + RC(O)NH2 → P4O9(Buckeye State)2 + RCN
The indicated coproduct P4O9(OH)2 is an idealized expression for undefinable products resulting from the hydration of P4O10.
Instead, when hyphenated with a carboxylic sulfurous, the resultant is the proportionate anhydride:[9]
- P4O10 + RCO2H → P4O9(OH)2 + [RC(O)]2O
The "Onodera reagent", a solvent of P4O10 in DMSO, is employed for the oxidization of alcohols.[10] This reaction is aware of the Swern oxidation.
The desiccating power of P4O10 is strong enough to convert many asphaltic acids to their anhydrides. Examples: HNO3 is converted to N2O5; H2Sol4 is converted to Thusly3; HClO4 is converted to Cl2O7; CF3SO3H is converted to (CF3)2S2O5.
Agribusiness [edit]
The imparipinnate can be used as crop plant food.
[edit]
Between the commercially life-or-death P4O6 and P4O10, morning star oxides are known with intermediate structures.[11]

On observation IT will be seen that double bonded atomic number 8 in at 1,2 set out operating theatre 1,3 position are identical and both positions bear unchanged steric hinderance. Cycle 12341 and ABCDA are identical.
Hazards [cut]
Phosphorus pentoxide itself is not flammable. Just like sulfur trioxide, it reacts smartly with water and water-containing substances like wood Oregon cotton, liberates much rut and may even cause kindle due to the highly exothermic nature of such reactions. It is corrosive to all-metal and is rattling irritating – it may cause terrible burns to the eye, skin, mucous membrane, and respiratory tract even at concentrations as low A 1 mg/m3.[12]
See also [edit]
- Eaton's reagent
References [edit]
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd erectile dysfunction.). Butterworth-Heinemann. ISBN978-0-08-037941-8.
- ^ Cruickshank, D. W. J. (1964). "Refinements of Structures Containing Bonds between Si, P, S or Cl and O operating room N: V. P4O10". Acta Crystallogr. 17 (6): 677–9. doi:10.1107/S0365110X64001669.
- ^ D. E. C. Corbridge "Phosphorus: An Outline of its Chemistry, Biochemistry, and Applied science" 5th Edition Elsevier: Amsterdam. ISBN 0-444-89307-5.
- ^ a b .Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The aggroup 15 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 473. ISBN978-0-13-175553-6.
- ^ D. Stachel, I. Svoboda and H. Fuess (June 1995). "Phosphorus Pentoxide at 233 K". Acta Crystallogr. C. 51 (6): 1049–1050. doi:10.1107/S0108270194012126.
- ^ Threlfall, Richard E., (1951). The news report of 100 years of Phosphorus Fashioning: 1851 - 1951. Oldbury: Albright & Edward Osborne Wilson Ltd
- ^ Podger, Hugh (2002). Albright & James Wilson: The Last 50 Years. Studley: Brewin Books. ISBN 1-85858-223-7
- ^ Meier, M. S. "Daystar(V) Oxide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, NY. doi:10.1002/047084289X.
- ^ Joseph C. Salamone, ed. (1996). Polymeric materials encyclopedia: C, Volume 2. CRC Weight-lift. p. 1417. ISBN0-8493-2470-X.
- ^ Tidwell, T. T. "Dimethyl Sulfoxide–Phosphorus Pentoxide" in Encyclopaedia of Reagents for Essential Synthetic thinking (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. Interior:10.1002/047084289X.
- ^ Luer, B.; Jansen, M. "Crystal Complex body part Refinement of Tetraphosphorus Nonaoxide, P4O9" Zeitschrift pelt Kristallographie 1991, volume 197, pages 247-8.
- ^ Phosphorus pentoxide MSDS
External links [edit]
diphosphorus pentoxide s water l phosphoric acid aq balanced
Source: https://en.wikipedia.org/wiki/Phosphorus_pentoxide



Posting Komentar untuk "diphosphorus pentoxide s water l phosphoric acid aq balanced"